- We ever fill the gap between hydrogen and helium on periodic table coin predictionSome people have already beaten me to the good answers, and I agree with th.
- Though Hydrogen atoms (H) turn into hydrogen molecules (H2) to form a very reactive, flammable, and explosive hydrogen gas, Helium (He) atoms form an inert gas. So, they never react, but may also result in reducing the hazard that pure hydrogen poses.
Vk download music for mac. Now let’s take a look at a couple of real systems. We begin by considering interactions between the simplest atoms, hydrogen (H) and helium (He), and the simplest molecule, molecular hydrogen (H2). A typical hydrogen atom consist of one proton and one electron, although some contain one or two neutrons and form “isotopes” known as deuterium and tritium, respectively. A hydrogen molecule is a completely different chemical entity: it contains two hydrogen atoms, but its properties and behavior are quite different. Helium atoms have 2 protons and 2 neutrons in their nuclei, and 2 electrons in their electron clouds. We will consider more complicated atoms and molecules after we discuss atomic structure in greater detail in the next chapter. One advantage of focusing on molecular hydrogen and helium is that it also allows us to introduce, compare, and briefly consider both van der Waals interactions (due to IMFs) and covalent bonds; we will do much more considering later on.
Helium is therefore a non-renewable natural resource, the price of which is constantly going up. Background. In spite of the fact that Hydrogen is also produced by various biological processes the atmosphere only contains 0,5 ppm Hydrogen, but as much as 5 ppm of Helium. Even if pure hydrogen gas has a low density and tend to rise. So that’s why, by mass, we say 75-76% was hydrogen and 24-25% was helium. But each helium nucleus is around four times the mass of a hydrogen nucleus, which means that, by number of atoms, the.
When two atoms of helium approach each other LDFs come into play and a attractive interaction develops. In the case of He the drop in potential energy due to the interaction is quite small, that is, the stabilization due to the interaction, and it does not take much energy to knock the two atoms apart. This energy is delivered by collisions with other He atoms. In fact at atmospheric pressures, Helium is never a solid and liquid He boils at ~4 K (−268.93ºC), only a few degrees above absolute zero or 0 K (−273.15 ºC).31 This means that at all temperatures above ~4 K there is enough kinetic energy in the atoms of the system to disrupt the interactions between He atoms. The weakness of these interactions means that at higher temperatures, above 4 K, helium atoms do not “stick together”. Helium is a gas at temperatures above 4 K.
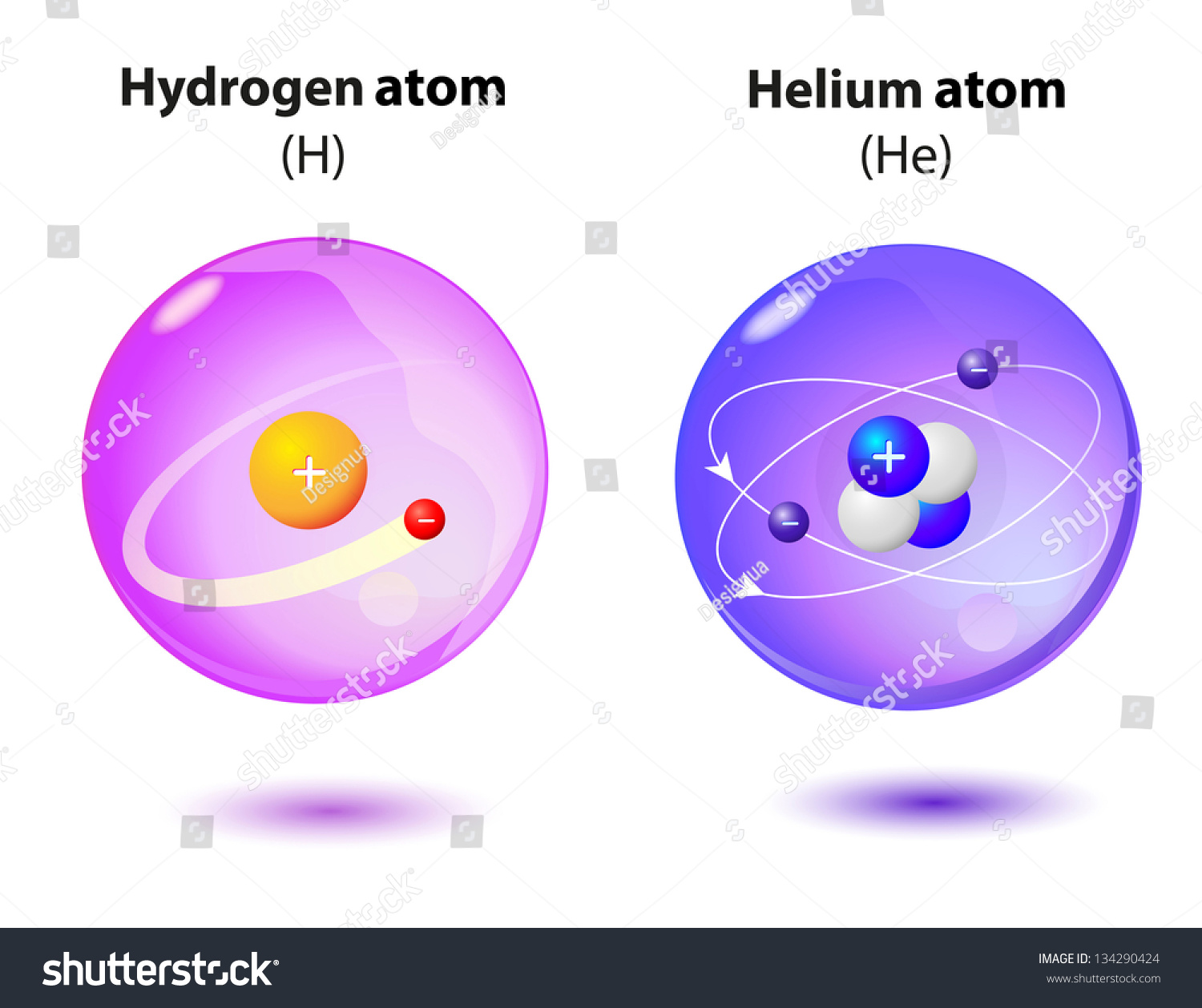
Now let us contrast the behavior of helium with that of hydrogen (H). As two hydrogen atoms approach one another they form a much more stable interaction, about 1000 times stronger than the He–He London dispersion forces. In an H–H interaction the atoms are held together by the attraction of each nucleus for both electrons. The attractive force is much stronger and as the atoms get closer this leads to a larger drop in potential energy and a minimum for the two interacting hydrogen atoms that is much deeper than that for He–He. Because of its radically different stability the H–H system gets a new name; it is known as molecular hydrogen or H2 and the interaction between the H atoms is known as a covalent bond. In order to separate a hydrogen molecule back into two hydrogen atoms, that is, to break the covalent bond, we have to supply energy.32 This energy can take several forms: for example, energy delivered by molecular collisions with surrounding molecules or by the absorption of light both lead to the breaking of the bond.
Fusion Of Hydrogen And Helium
Each H can form only a single covalent bond, leading to the formation of H–H molecules, which are often also written as H2 molecules. These H–H molecules are themselves attracted to one another through LDFs. We can compare energy associated with the H–H covalent bond and the H2 - H2 IMF. To break a H–H covalent bond one needs to heat the system to approximately 5000 K. On the other hand to break the intermolecular forces between separate H2 molecules, the system temperature only needs to rise to ~20 K; above this temperature H2 is a gas. At this temperature the IMFs between individual H2 molecules are not strong enough to resist the kinetic energy of colliding molecules. Now you may ask yourself, why does H2 boil at a higher temperature than He? Good question! It turns out that the strengths of LDFs depend on several factors including shape of the molecule, surface area, and number of electrons. For example the greater the surface areas shared between interacting atoms or molecules the greater the LDFs experienced and the stronger the resulting interaction. Another factor is the ability of the electron cloud to become charged, a property known as polarizability. You can think of polarizability as the floppiness of the electron cloud. As a rough guide, the further away from the nucleus the electrons are, the more polarizable (floppy) the electron cloud becomes. We will return to this and related topics later on. As we will see, larger molecules with more complex geometries, such as biological macromolecules (proteins and nucleic acids), can interact through more surface area and polarizable regions, leading to correspondingly stronger interactions.
At this point, you are probably (or should be) asking yourself some serious questions, such as, why don’t helium atoms form covalent bonds with one another? Why does a hydrogen atom form only one covalent bond? What happens when other kinds of atoms interact? To understand the answers to these questions, we need to consider how the structure of atoms differs between the different elements, which is the subject of the next chapter. Tunecrack.
Questions to Answer
- Can you draw a picture (with about 20 helium atoms, represented as circles) of what solid helium would look like if you could see it?
- How would that differ from representations of liquid helium or gaseous helium?
- Now make a similar drawing of H2. Does this help explain the higher melting point of (H_2)?
Questions to Ponder

- 1. How do the properties of solids, liquids, and gases differ?
Helium And Hydrogen Compound
References

31According to Robert Parson, “ At 1 atmosphere pressure, Helium does not melt at ANY temperature - it stays liquid down to absolute zero. (If you want to be picky, it is a liquid down to the lowest temperatures that anyone has ever achieved, which are orders of magnitude less than 1 K (http://en.wikipedia.org/wiki/Dilution_refrigerator), and our best theories predict that it will remain a liquid no matter how low the temperature.) To get solid helium you have to increase the pressure to 25 atmospheres or above. This is one of the most dramatic consequences of zero-point energy: the intermolecular forces in He are so weak that it melts under its own zero point energy. (This leads to the peculiar consequence that Helium at zero Kelvin is a liquid with zero entropy.)
Helium And Hydrogen On Periodic Table
32 In fact this is known as the bond energy – the energy required to break the bond – which in the case of H2 is 432 kJ/mol.
